How Many Atoms Are in a Mole of Al2o3
One mole of molecular compound contains 602 x 1023 molecules d. Compare a mole of Ag-108 and a mole of Pt-195 using atoms protons electrons and neutrons.

How To Find The Number Of Atoms In Al2o3 Aluminum Oxide Youtube
How many atoms of potassium does 1 mol of potassium contain.
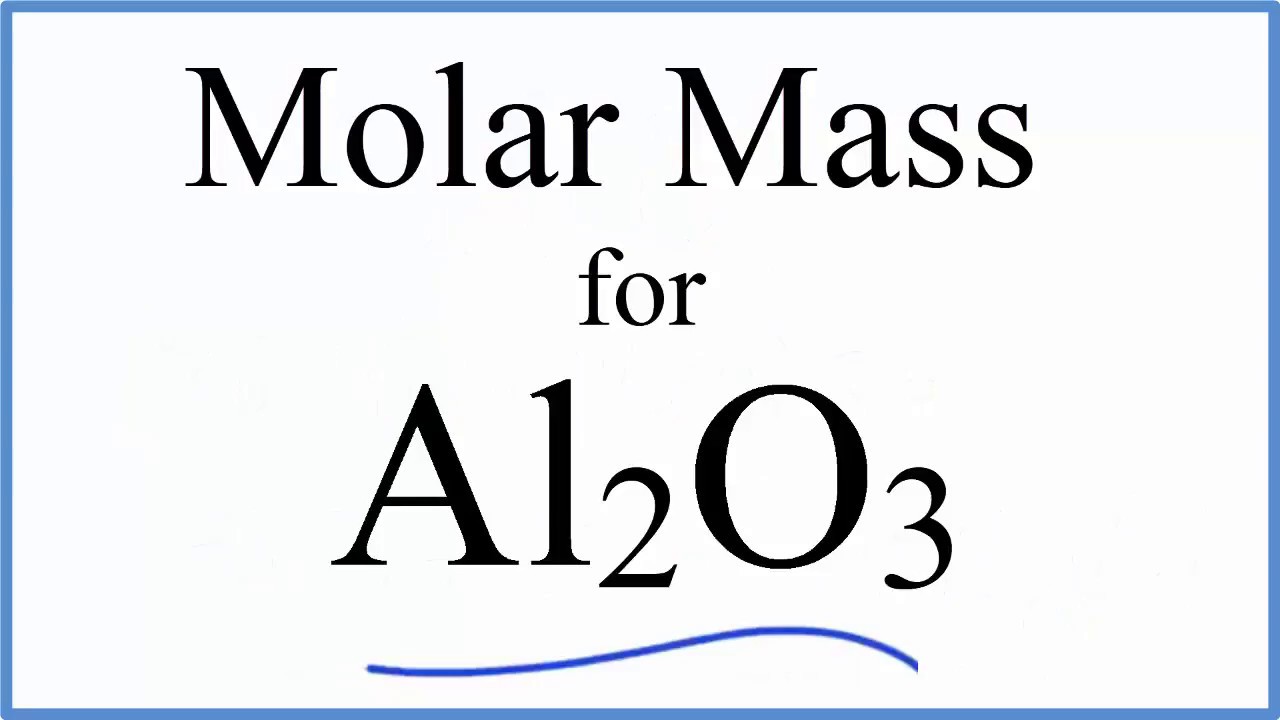
. Calculate 1 partial pressures 2 mole fractions 3 Concentrations 4 densities of the A. Which of the following samples contains 40 moles of O atoms. More specifically a mole is a pile that contains 6022 x 1023 particles or objects.
Since they both contain one mole you have 602 x 10 23 particles of silver and 602 x 10 23 particles of platinum. One mole of ionic compound contains 602 x 1023 cations and 602 x 1023 anions 129 130. What volume of water w.
1mole of al2so43 contains 12 mole oxyzen atom. 602 x 10 23 potassium atoms. Finding molar mass starts with units of grams per mole gmol.
When calculating molecular weight of a chemical compound it tells us how. If you begin with 1976 grams of Als and 1514 grams of O2g how many grams of Al2O3s. Mole Wilhelm Ostwald LatvianGerman chemist and a 1909 Nobel prize laureate.
A mole is a counting number that means 6022 1023 just as a dozen means 12. You are asked to determine the alkali present and. 10 mole SO3 d.
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. 20 moles SO2 c. The structure of the transitional Al2O3 γ vs.
δ played a smaller role than Na contamination in detg. Separation Process Principles- Chemical and Biochemical Operations 3rd Edition. A mole of oxygen O2 contains the same number of atoms as a mole of methane CH4.
36 mol C atoms 72 mol H atoms 36 mol O atoms. 1 mol Al2O3 1 mol Al2CO33. Then 1 mole of oxygen atom is in 112mole of aluminium solphate.
It has been shown in the literature that many factors including the particle size shape chemical composition metalsupport interaction and metalreactantsolvent interaction can have significant. How many atoms are in 070. 1 mole 602 x 1023 atoms monoatomic element 1 mole molar mass grams - HOW HEAVY.
18531932 introduced the word mole in 1896 as follows one mole as molecular weight of a substance in grams In 1969 International Committee on Weights and Measures defined the mole as the amount of a substance that contains as many elementary entities as there are. 1 mole 224 L for a gas at STP - HOW MUCH SPACE. Click to see the answer.
10 mole SO2 b. Covalent 1 mole 602 x 1023 formula units ionic HOW MANY PARTICLES. How many moles of atoms are in 216 mol of Al2O3.
How many moles of carbon atoms hydrogen atoms and oxygen atoms are present in a 12-mole sample of lactic acid. One mole of an element is numerically equal to its atomic mass unit. The difference would be in the number.
How many moles of H atoms are present in one mole of H3pO4 molecules. Metal species with different size single atoms nanoclusters and nanoparticles show different catalytic behavior for various heterogeneous catalytic reactions. How many atoms of hydrogen are in 626 L of H2 at STP.
In a steady-state flow reactor α-Al2O3 was 1000 times less reactive in terms of moles propene formedm2 s than reforming-grade γ-Al2O3. One mole of an element contains 602 x 1023 atoms c. With this in mind we can now create a conversion factor that can be used to determine the number of particles in a mole or pile or vice versa.
Click to see the answer. It can be said that a. The effect of Al2O3 form γ δ and α and impurities on Me2CHOH dehydration activity was studied.
22 mol Al2O3 x 1 mol Al2CO33 x 234 g Al2CO33 5148 g Al2O3. Then 025 mole of oxygen atom is in 02512 mole of aluminum solphate. These particles are typically atoms molecules or ions.

How To Find The Number Of Atoms In Al2o3 Aluminum Oxide Youtube

Molar Mass Molecular Weight Of Al2o3 Aluminum Oxide Youtube

Comments
Post a Comment